21 February 2012
4QCY12 Results Update | Sector: Healthcare
GlaxoSmithKline Pharmaceuticals
BSE SENSEX
S&P CNX
18,429
5,607
CMP: INR2,088
TP: INR2,272
Buy
Bloomberg
GLXO IN
Equity Shares (m)
84.7
52-Week Range (INR) 2,475/1,830
1,6,12 Rel. Perf. (%)
-4/-11/-5
M.Cap. (INR b)
176.9
M.Cap. (USD b)
3.6
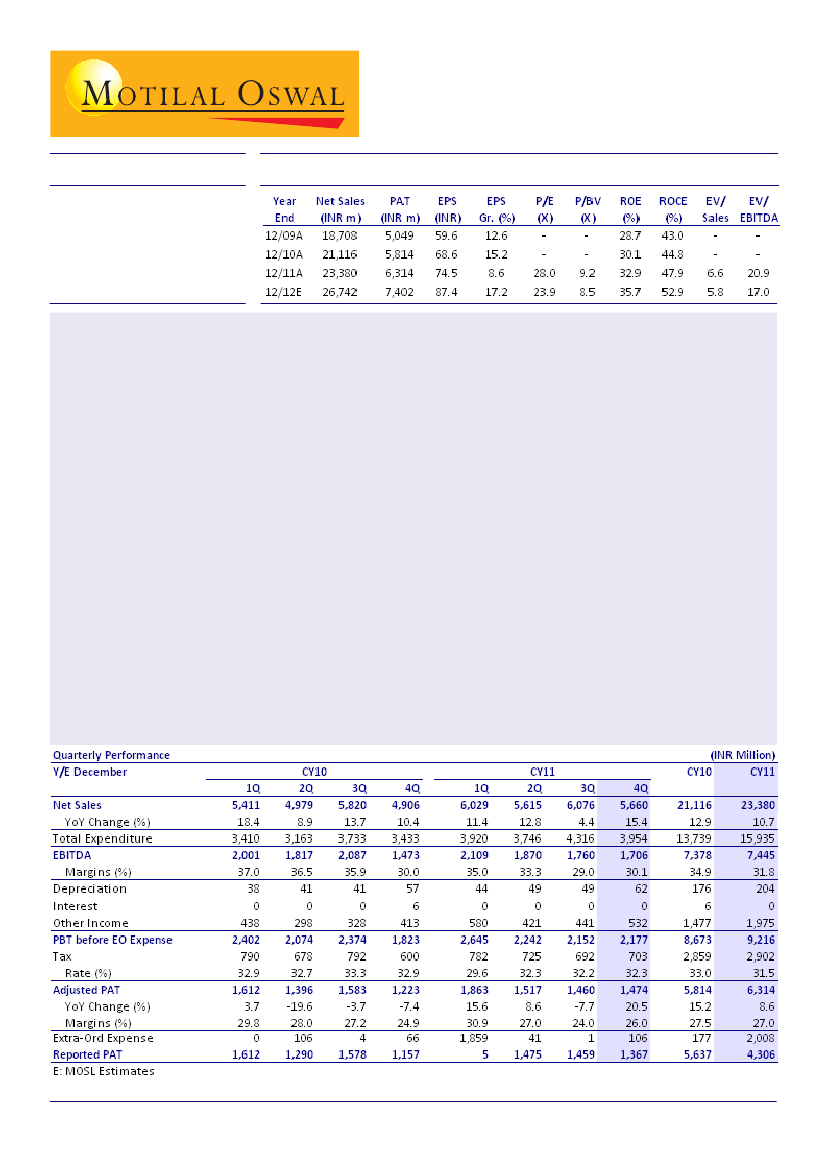
GlaxoSmithKline Pharmaceuticals (GLXO) posted net sales of INR5.66b (v/s our estimate of INR5.43b) for
4QCY12, up 15.4% YoY. EBITDA grew 15.8% YoY to INR1.71b (v/s our estimate of INR1.62b) while adjusted PAT
increased 20.5% YoY to INR1.47b (v/s our estimate of INR1.3b).
While overall revenue growth was 15.4% YoY, revenue from the core pharmaceuticals business grew by an
impressive 18.2% YoY. Topline growth was led by revival in the Mass Market business and Anti-infectives
segments. Vaccines and Specialty segments including Oncology, Dermatology, Cardiovascular and Metabolics
reported robust double-digit growth, driven by new product launches.
EBITDA grew 15.8% YoY to INR1.71b; EBITDA margin remained flat YoY at 30.1% (v/s our estimate of 29.8%).
EBITDA was higher than estimated, primarily led by better than estimated topline growth.
Adjusted PAT increased by 20.5% YoY to INR1.47b (v/s our estimate of INR1.3b), led by higher than estimated
other income and lower effective tax rate. GLXO has declared INR45/share as dividend.
We believe GLXO is one of the best plays on the IPR regime in India, with aggressive plans to launch new products
in the high-growth lifestyle segments. These launches should bring in long-term benefits. While we expect
double-digit topline growth to sustain over the next few years, the growth trajectory should improve further
post CY13, as new launches start contributing meaningfully. This growth is likely to be funded through miniscule
capex and negative net working capital. GLXO deserves premium valuations due to strong parentage (giving
access to large product pipeline), brand-building ability and likely positioning in the post-patent era. It is one of
the very few companies, with the ability to achieve reasonable growth without any major capital requirement,
leading to high RoCE of over 45%. We expect GLXO to record an EPS of INR87.4 (up 17.2%) in CY12. The stock
currently trades at 23.9x CY12E EPS. Maintain
Buy,
with a target price of INR2,272 (26x CY12E EPS).
Nimish Desai
(NimishDesai@MotilalOswal.com); Tel: +91 22 39825406
Amit Shah
(Amit.Shah@MotilalOswal.com); Tel: + 91 22 3982 5423