7 February 2013
3QFY13 Results Update |
Sector: Healthcare
Cipla
BSE Sensex
19,640
Bloomberg
Equity Shares (m)
M.Cap. (INR b)/(USD b)
52-Week Range (INR)
1,6,12 Rel. Perf. (%)
S&P CNX
5,959
CIPLA IN
802.9
325.2/6.1
435/287
-4/4/4
CMP: INR405
TP: INR452
Neutral
Financials & Valuation (INR b)
Y/E March
Sales
EBITDA
Net Profit
Adj. EPS (INR)
EPS Gr. (%)
BV/Sh. (INR)
RoE (%)
RoCE (%)
Payout (%)
Valuations
P/E (x)
P/BV (x)
EV/EBITDA (x)
Div. Yield (%)
2013E 2014E 2015E
83.0
22.0
13.5
16.8
20.2
110.5
15.2
22.6
20.0
24.0
3.7
14.8
0.8
89.7
22.4
15.8
19.7
16.8
125.2
15.7
20.7
25.0
20.6
3.2
14.5
1.0
102.6
25.6
18.2
22.6
14.9
142.2
15.9
21.4
25.0
17.9
2.8
12.7
1.2
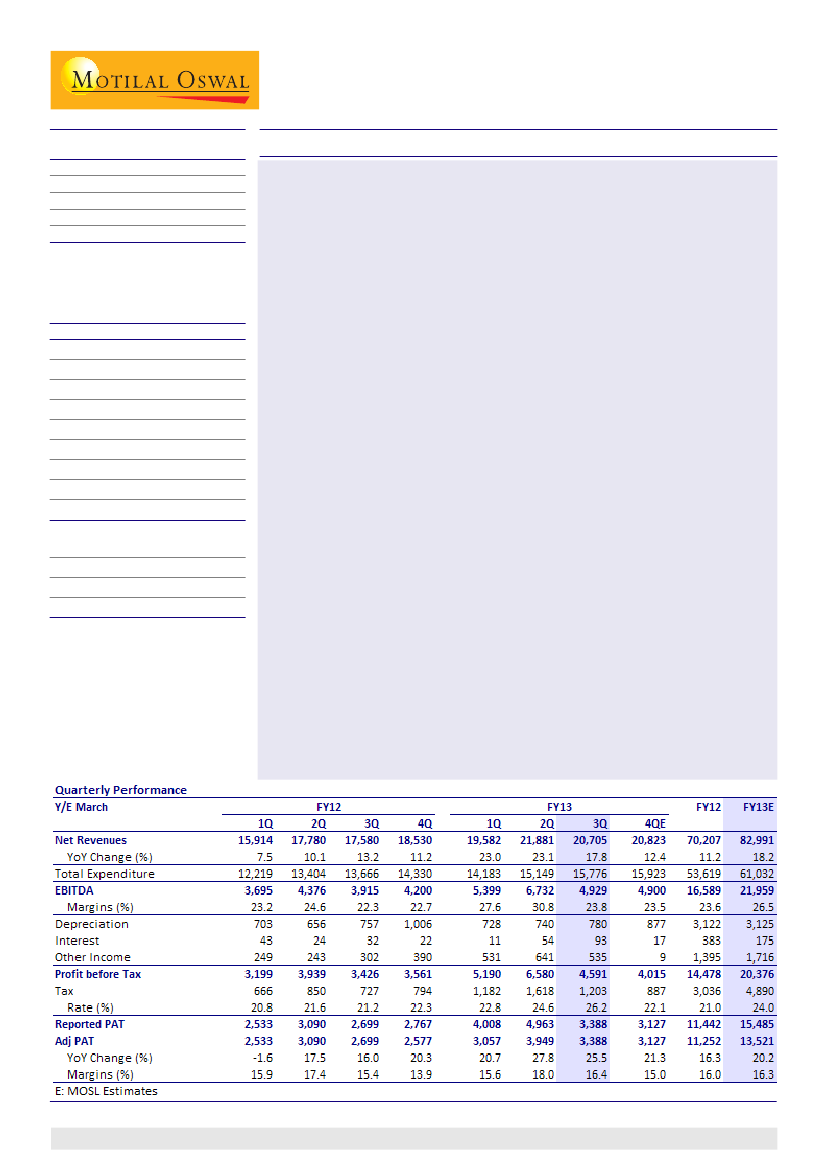
Revenues grew 18% YoY to INR20.71b (v/s est INR20.17b), EBITDA grew 26%
YoY to INR4.93b (v/s est INR4.71b) and PAT was up 26% to INR3.39b (v/s est
INR3.25b). Top line growth was primarily led by 38% YoY growth in export
formulations, while domestic formulations grew a modest 10% YoY. Export
APIs stood at INR1.38b and declined by 16% YoY on a high base.
We are positively surprised by the strong growth in export formulations,
driven by Lexapro supplies (now part of the base business) and strong growth
witnessed across key therapeutic areas. Also, there was a 6-7% benefit from
currency; however, management indicated that this high growth is not
sustainable, going forward. The muted domestic formulations growth of 10%
is below our expectation of 15%.
EBITDA margin increased 150bp YoY and stood at 23.8% (v/s our est of 23.3%).
Margin improvement was driven by favorable product mix, with higher
contribution from anti-depressants segment (includes generic Lexapro
supplies, now part of base business) and anti-allergics (includes Dymista,
currently a small contributor).
PAT growth was aided by strong top line growth coupled with healthy
operational performance. There was a forex gain of INR190m for the quarter.
While export formulations grew 38% YoY in 3QFY13, Cipla's core quarterly
performance has not been encouraging in the past many quarters. Its muted
export performance had raised uncertainty on the timelines of ramp-up at Indore
SEZ. We believe it is imperative for the company to improve asset utilization at
Indore to drive future growth and derive benefits of operating leverage
(overhead expenses continue to adversely impact performance). Post 3QFY13
performance, our core EPS est for FY13E has witnessed an upgrade of 2%, while
FY14E/15E est are largely unchanged. Our revised est take into account better-
than-expected performance in export formulations during this quarter, partially
offset by the muted growth in domestic formulations. The stock trades at 20.6x
FY14E and 17.9x FY15E EPS. Maintain
Neutral
with a TP of INR452 (20x FY15E EPS).
Hardick Bora
(Hardick.Bora@MotilalOswal.com)
Investors are advised to refer through disclosures made at the end of the Research Report.
1