30 January 2016
3QFY16 Results Update | Sector: Healthcare
BSE SENSEX
24,871
Bloomberg
Equity Shares (m)
M.Cap. (INR b)/ USD
b)
52-Week Range (INR)
1, 6, 12 Rel.Per (%)
AvgVal,(INR m)
Free float (%)
S&P CNX
7,564
GNP IN
271.3
209.2/3.1
1,262/705
-13/-12/23
1,042
53.5
Glenmark Pharma
CMP: INR771
TP: INR900 (+17%)
Neutral
Results below estimates; LatAm and US underperform
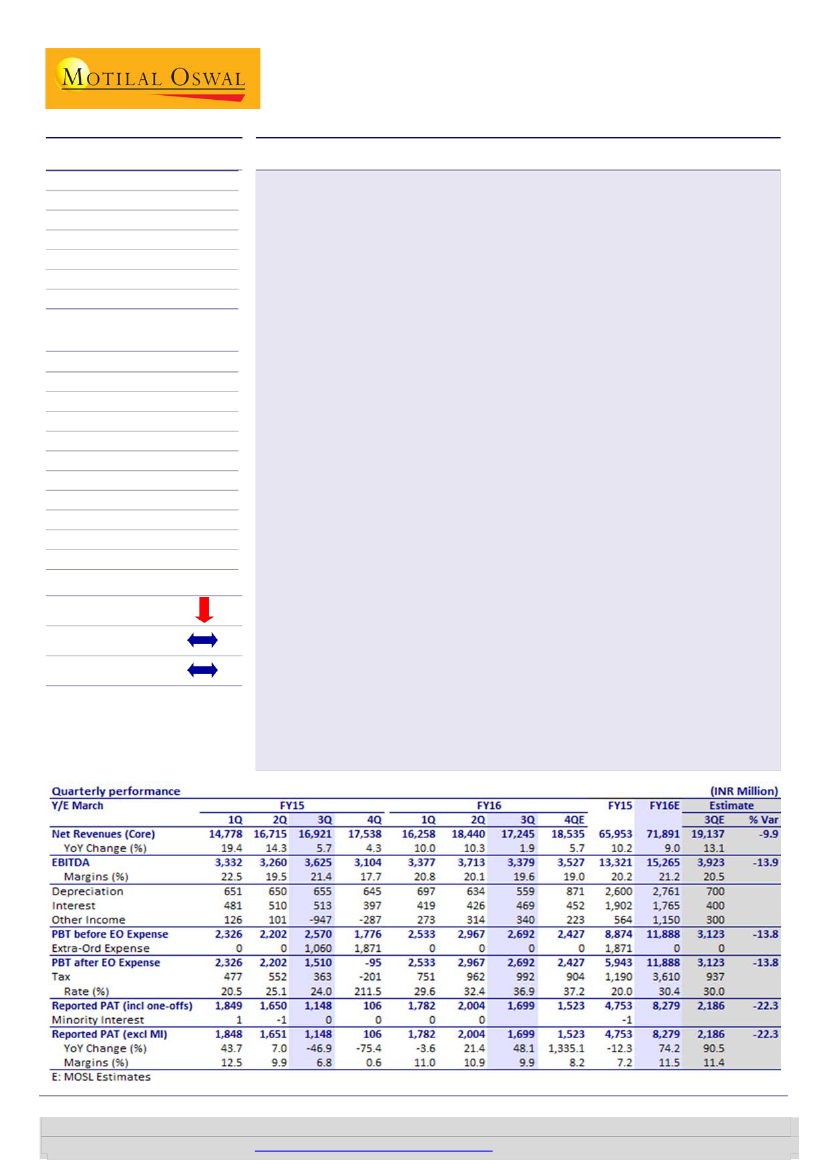
Glenmark’s (GNP) 3QFY16 performance was largely below our estimates. Revenue
grew 2% YoY (10% miss) and EBITDA 7% YoY (14% miss). PAT stood at INR1.7b (48%
growth; 22% miss).
Latam and weak US affect 3Q:
US business grew 20%YoY to INR6.1b (6% miss) on
the back of continued pricing pressure on existing portfolio and lower traction in
newly launched products during the quarter. Stoppage of supplies to Venezuela
market and steep depreciation in Brazilian currency resulted in LatAm market sales
declining 47% YoY to INR1.2b (24% miss) in 3Q. India business reported 12.7 %YoY
growth on account of loss of sales of Sitagliptin products. Despite forex headwinds
in Russia/CIS region, SRM segment grew 14% YoY (in line). We estimate GNP to
post 16% revenue CAGR over FY15-18 (v/s 10% in FY15).
EBITDA margin disappoints:
EBITDA margin at 19.6% was 90bp below our
estimates on higher other expenses in 3Q. Other expenses at 31% of sales were
300bp higher than our estimates, mainly on account of negative operating leverage
on lower sales. Surprisingly, gross margin at 70.6% was 350bp higher than 3QFY15.
R&D spends stood at INR1.7b in 3Q, 9.2% of sales. Going forward, we expect
margin to improve from 21.4% in FY16 to 25% in FY18 on the back of improved
operating leverage and niche product launches in the US.
Earnings call highlights:
1) Venezuela sales are likely to become negligible going
ahead. 2) Gross debt increased to INR34.5b in Dec’15 from INR32b in Sep’15 on the
back of Tarka liability (INR2.1b). 2) Net forex losses stood at INR270m in 3Q. 3) The
company doesn’t anticipate any AG product launch with Zetia. 4) GNP has signed
50:50 profit share agreement with its partner for Zetia.
Maintain Neutral
:
Post the recent correction in stock price (down ~20% in the
last one month), valuations are not expensive. Having said that, currency volatility
in emerging markets and potential risk of write-off of cash in Venezuala (~USD35m)
will keep multiples range-bound. We have cut our FY16-18E EPS by 6-9% as we
build zero sales from Venezuala going forward. Though Zetia exclusivity will bring
debt under check, pick-up in US base business is the key to maintain sustainable
growth. Maintain
Neutral
(TP: INR900, 18x on FY18E EPS + INR20 NPV for Zetia).
Big In-licensing deal in innovation business could act as a positive catalyst.
Financials & valuations (INR b)
Y/E Mar
Sales
EBITDA
Net Profit
AdjEPS(INR)
)
EPS Gr. (%)
BV/Sh(INR)
RoE (%)
RoCE (%)
P/E (x)
P/BV (x)
2015
66.0
11.8
4.8
17.5
-12.3
110.6
15.8
14.4
44.0
7.0
2016E 2017E
71.9
15.4
8.4
29.8
70.4
17.9
18.6
25.8
4.6
87.9
23.4
11.3
39.9
33.7
18.6
26.6
19.3
3.6
166.4 214.5
Estimate change
TP change
Rating change
Kumar Saurabh
(Kumar.Saurabh@MotilalOswal.com); +91 22 3982 5584
Amey Chalke
(amey.chalke@motilaloswal.com); +91 22 39825423
Investors are advised to refer through important disclosures made at the last page of the Research Report.
Motilal Oswal research is available on
www.motilaloswal.com/Institutional-Equities,
Bloomberg, Thomson Reuters, Factset and S&P Capital.