12 February 2016
Q3FY16 Results Update | Sector: Healthcare
Sun Pharma
Buy
BSE SENSEX
22,986
Bloomberg
Equity Shares (m)
M.Cap.(INRb)/(USDb)
52-Week Range (INR)
1, 6, 12 Rel. Per (%)
Avg Val, (INR m)
Free float (%)
S&P CNX
6,981
SUNP IN
2,406.6
2,041.6 / 29.9
1,201/706
14/12/12
4,590
45.3
CMP: INR848
TP: INR975(+15%)
Strong results; multiple triggers to drive growth
Financials & Valuation (INR b)
Y/E Mar
2016E 2017E 2018E
Sales
283.0 336.0 361.6
EBITDA
82.5 111.8 128.9
Adj. PAT
47.8
69.6
93.8
Core EPS (INR)
19.9
28.9
39.0
EPS Gr. (%)
0.9
66.3
18.0
BV/Sh. (INR)
120.3 146.3 178.3
RoE (%)
17.5
21.7
24.0
RoCE (%)
21.8
30.0
30.8
P/E (x)
42.7
29.3
21.7
P/BV (x)
7.1
5.8
4.8
Estimate change
TP change
Rating change
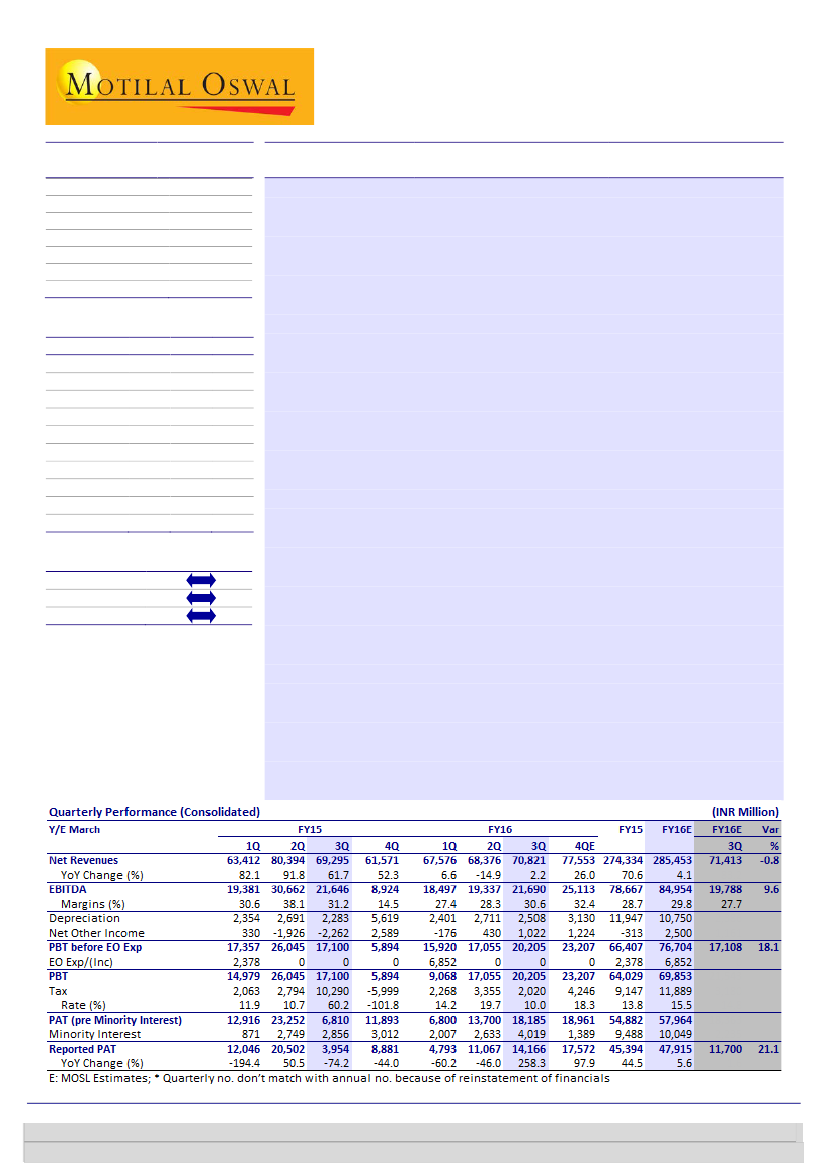
SUNP’s reported sales of INR70.8bn (in-line with est) with better than expected
EBITDA margins of 30.6% (vs est of 28%) and PAT of INR14.2b (vs INR11.1b in 2Q).
Positive surprise on profitability is on the back of better product mix, other
operating income and lower tax. We expect EPS for SUNP to double by FY18 to
INR39 (v/s INR20 in FY15)— primarily on the back of limited competition for
generic Gleevec, RBXY integration benefits, ramp-up of specialty products (Keveyis/
Xenazine/ Xelpros/ Elepsia XR) and mid-teens growth in India branded business.
US business- Gleevec to drive near term growth:
US sales stood at USD486m in 3Q
and were down 5% QoQ primarily due to one-time sales of ~USD40m in 3Q
partially offset by strong Taro performance (up 22% QoQ). Management plans to
complete the remediation measure shortly and will be asking US FDA for re-
inspection in 1Q FY17E. Currently we have built resolution by 2H FY17. We expect
US sales growth in medium term to be driven by Gleevec FTF, ramp-up of speciality
business products (Xenazine, Keveyis, etc).
Domestic business- recovery in growth to continue.
Domestic business revenue
grew at ~8% YoY. Growth was impacted due to withdrawal of bonus offers in the
acute segment. Having said that, revenue growth improved sequentially in 3Q (vs
1% YoY in 2Q) as the impact of promotional cuts moderated. According to AIOCD,
secondary sales growth was at ~10% YoY in 3Q signaling better growth ahead.
Earnings call takeaways:
(a)MK-3222 Phase-3 data to come out by April,(b)
Gleevec market share expected to reach ~30% during FTF, (c) Halol remediation is
on track and expects re-inspection in near term, (d)MK-3222 is likely to be filed in
CY17, (e)SUNP is on track to achieve USD300m Ranbaxy synergy by FY18E.
Multiple triggers, coupled with recent weakness, provide valuation upside:
Sun
Pharma is one of our top picks in Indian pharma with TP of INR975 @ 25x FY18E
P/E (@10% discount to historical average). Our buy rating is based on the back of
multiple triggers (MK-3222 Phase-3 data, Gleevec launch, RBXY integration benefits
and Keveyis ramp-up), superior execution track record, high RoIC (30%) and cash-
rich balance sheet (USD1b net cash). Post strong 4Q numbers, we have increased
our FY16 EPS by 5-6%. We estimate EPS CAGR of 26% over FY15-18 despite hike in
R&D and tax rate (tax rate @ 20% in FY18 v/s 14% in FY15).
Investors are advised to refer through important disclosures made at the last page of the Research Report.
Motilal Oswal research is available on www.motilaloswal.com/Institutional-Equities, Bloomberg, Thomson Reuters, Factset and S&P Capital.
Kumar Saurabh
(Kumar.Saurabh@MotilalOswal.com); +91 22 3982 5584
Amey Chalke
(Amey.Chalke@MotilalOswal.com); +91 22 39825423