Ipca Laboratories
BSE SENSEX
31,159
Bloomberg
Equity Shares (m)
M.Cap.(INRb)/(USDb)
52-Week Range (INR)
1, 6, 12 Rel. Per (%)
Avg Val, INRm
Free float (%)
S&P CNX
9,625
IPCA IN
Weak quarter; resolution of FDA issue key
126
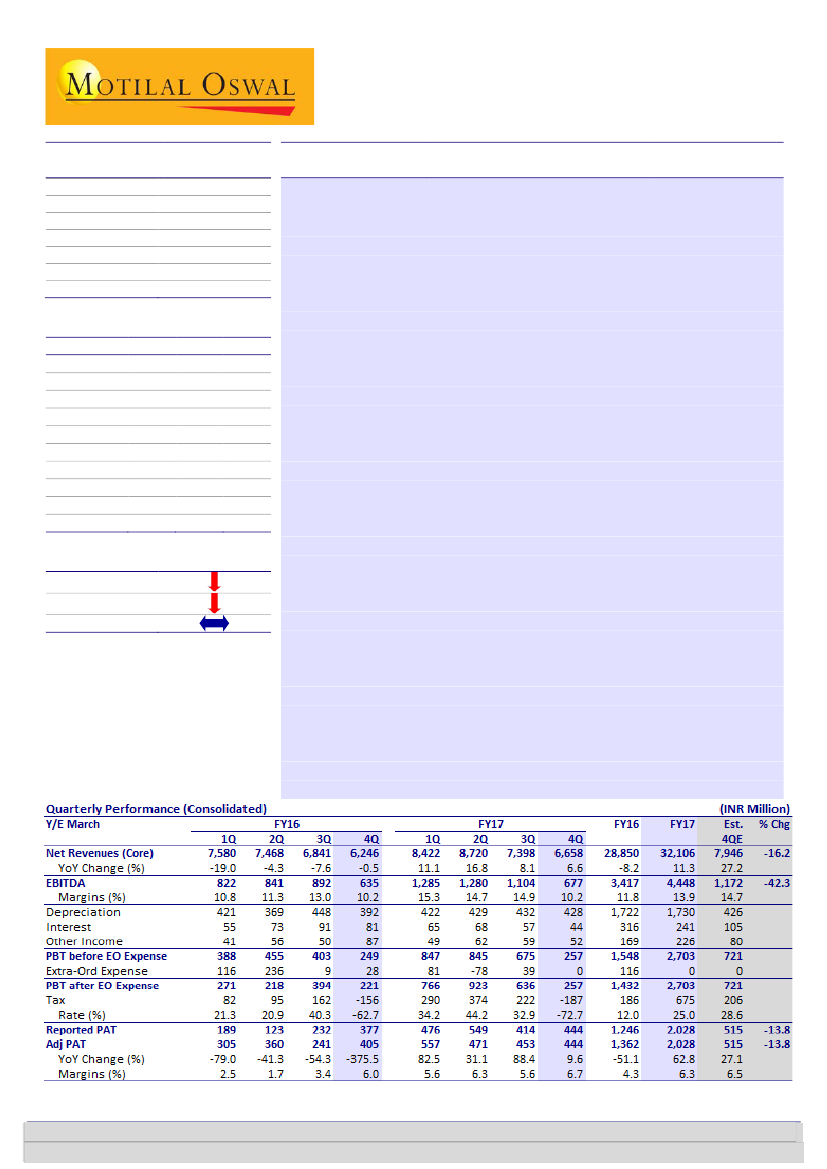
4QFY17 revenue of INR6.7b (+7% YoY) came in 16% below our estimate,
67.7 / 1.1
primarily due to muted performance by the generic and domestic
656 / 402
businesses. EBITDA margin contracted significantly to 10.2% (-480bp QoQ
-22/-32/-6
and flat YoY) due
to muted sales and lower gross margin. Reported PAT of
119
INR444m included the impact of deferred tax asset of INR237m in 4Q. For FY17
53.9
30 May 2017
4QFY17 Results Update | Sector: Healthcare
CMP: INR489
TP: INR480(-2%)
Neutral
Financials & Valuations (INR b)
2017 2018E
Y/E Mar
Net Sales
32.1
35.0
EBITDA
4.4
5.4
PAT
2.0
2.8
EPS (INR)
16.1
22.4
Gr. (%)
52.8
39.4
BV/Sh (INR)
194.1
213.1
RoE (%)
8.6
11.0
RoCE (%)
7.4
9.5
P/E (x)
30.4
21.8
P/BV (x)
2.5
2.3
2019E
40.6
6.7
3.8
29.9
33.5
238.5
13.2
11.4
16.3
2.0
Estimate change
TP change
Rating change
sales, EBITDA and Adj. PAT stood at INR32b (+11%YoY), INR4.4b (+30%YoY) and
INR2b (+53%YoY) respectively
Domestic/generic businesses deliver muted performance:
India formulation
sales grew ~11% YoY to ~INR2.8b. India business growth was impacted by the
demonetization drive. Apart from this, NLEM and FDC ban impacted domestic
revenue growth by ~4% and 2%, respectively. Management expects domestic
business to grow at ~12-14% YoY in FY18 as the impact of demonetization is
already fading. Export formulations segment declined ~10% YoY due to a fall in
generics business by ~16% YoY to INR1b and in institutional business by 34% YoY
to INR310m. Generic business declined due to weak EU sales growth.
Update on regulatory resolution:
IPCA management is planning to meet USFDA
in Jun-17 to offer invitation for Piparia inspection. Invite for Pithampur
inspection is expected by mid-3Q. Currently >90% of ANDAs are linked to API
from Ratlam. Given that the remediation process at Ratlam will only get over by
end-CY17, we do not expect full regulatory resolution before 2HFY19E.
Key earnings call takeaways:
a) R&D as % of sales expected to be ~4% in FY18E.
b) Filed 1 new ANDA in FY17; total 43 ANDA filings – 2 in market, 25 pending
and 18 approved. c) Debt of INR7.2b at FY17 end; d) Received RFP from global
funds for institutional business tender scheduled at 2018 beginning; expected to
participate without USFDA resolutions.
Resolution of regulatory issues key:
At CMP, the stock trades at 21.8x FY18E
EPS, at a discount to three-year average P/E. We do not expect a revival in US
business before 2HFY19E. Despite the stock’s attractive valuation, we believe
regulatory overhang will keep multiples under check in the near term. Reiterate
Neutral
on IPCA with a TP of INR480 @ 16x FY19E, discount of ~15-20% to its
peers (v/s INR540 @ 16x 1H FY19E). We have cut our FY18/19E EPS by ~19% as
we build in delay in USFDA resolution and muted margins in base business.
Investors are advised to refer through important disclosures made at the last page of the Research Report.
Motilal Oswal research is available on www.motilaloswal.com/Institutional-Equities, Bloomberg, Thomson Reuters, Factset and S&P Capital.
Kumar Saurabh
(Kumar.Saurabh@MotilalOswal.com); +91 22 6129 1519