Ipca Laboratories
BSE SENSEX
32,942
Bloomberg
Equity Shares (m)
M.Cap.(INRb)/(USDb)
52-Week Range (INR)
1, 6, 12 Rel. Per (%)
Avg Val, INRm
Free float (%)
S&P CNX
10,187
IPCA IN
Strong recovery in margins
126
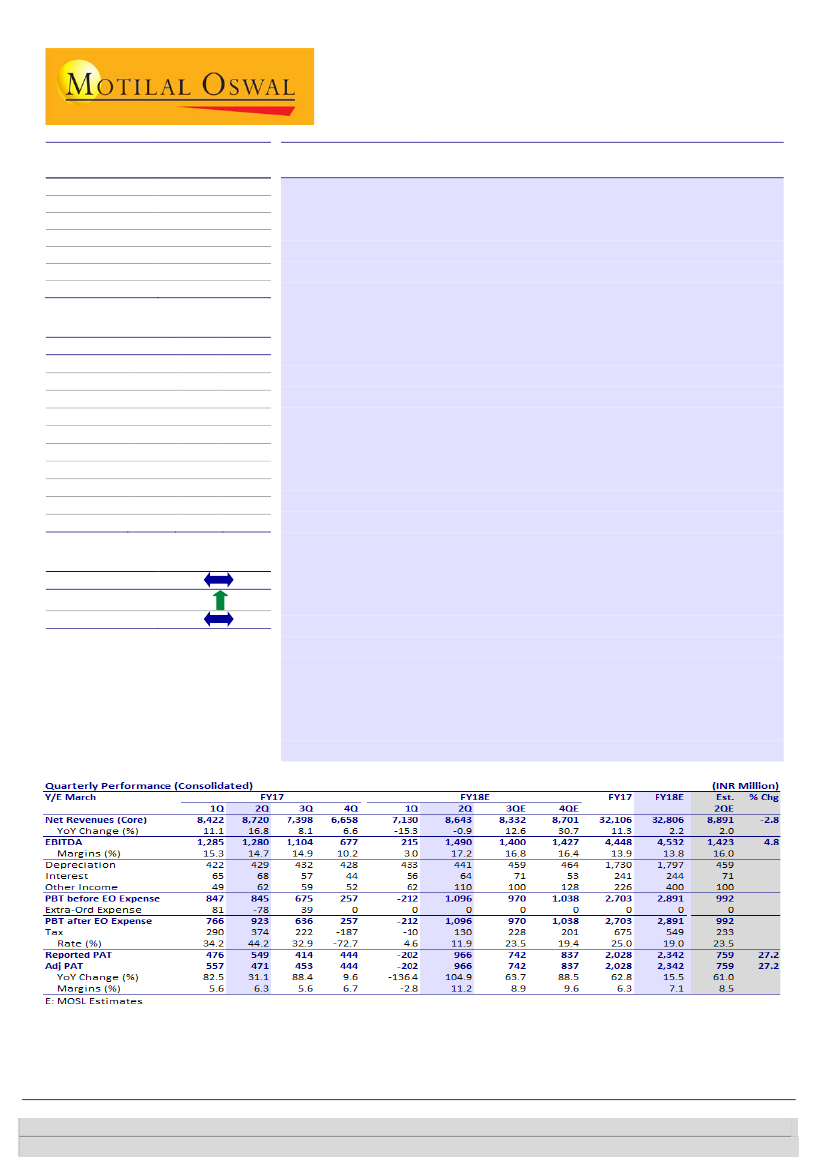
2QFY18 revenue was flat YoY at INR8.6b (~3% below est.). Domestic
67.6 / 1.0
business was up 3% YoY. Gross margin expanded to 65.9% from 62.5% in
656 / 400
2QFY17. EBITDA rose ~16% YoY to INR1.5b (+5% beat), with the margin
6/-9/-27
128
improving to 17.2% (+260bp YoY). EBITDA margin saw a negative impact of
53.9
~300bp from GST rollout. PAT rose 105% YoY to INR966m (+27% beat).
14 November 2017
2QFY18 Results Update | Sector: Healthcare
CMP: INR536
TP: INR550(+3%)
Neutral
Financials & Valuations (INR b)
2017 2018E
Y/E Mar
Net Sales
32.1
32.8
EBITDA
4.4
4.5
PAT
2.0
2.3
EPS (INR)
16.1
18.6
Gr. (%)
52.8
15.5
BV/Sh (INR)
194.6
210.3
RoE (%)
8.6
9.2
RoCE (%)
7.5
8.2
P/E (x)
33.3
28.9
P/BV (x)
2.8
2.5
2019E
37.0
6.0
3.4
26.5
43.1
232.9
12.0
10.5
20.2
2.3
Estimate change
TP change
Rating change
Domestic business (ex anti-malaria) delivers strong growth:
India
formulation sales grew 5% YoY to ~INR4.2b. According to management,
domestic branded business (ex anti-malaria) grew ~23-24% YoY. IPCA lost
~INR350-400m of sales v/s 2QFY17 due to lower Malaria-related sales.
Management expects growth to bounce back to low-to-mid-teens in coming
quarters, led by channel re-filling. International generic revenue fell ~18%
YoY, mainly on weak UK/flat US business. Institutional business reported
revenue of INR220m, as ~INR150 of sales got deferred to 3QFY18.
Pick-up in institutional business from CY18E:
IPCA announced that Global
Funds has selected the company as its panel supplier. At peak, institutional
biz revenue was ~INR4b. Adjusted for price deflation, peak business should
be ~INR2.5b (v/s IPCA’s current run-rate of ~INR1.2-1.3b). IPCA expects to
reach peak sales in FY19. Two more approvals in anti-malaria are expected
in FY19, which could add ~INR1.5b to top-line in two years.
Update on regulatory resolution:
IPCA has already invited USFDA for
Piparia/Pithampur plants inspection. Currently, >90% of ANDAs are linked to
API from Ratlam. IPCA plans to invite the USFDA for re-inspection at Ratlam
plant by CY17 end/CY18 beginning. We do not expect a pick-up in US
business in FY19 as resolution at these plants can take at least 6-12 months.
Resolution of regulatory issues is key:
Reiterate
Neutral
with a TP of INR550
@ 18x 1HFY20E PER (v/s INR430 @ 16x FY19E). We have increased our
target multiple on the back of the improved business outlook.
Investors are advised to refer through important disclosures made at the last page of the Research Report.
Motilal Oswal research is available on www.motilaloswal.com/Institutional-Equities, Bloomberg, Thomson Reuters, Factset and S&P Capital.
Kumar Saurabh-Research analyst
(Kumar.Saurabh@MotilalOswal.com); +91 22 6129 1519
Ankeet Pandya-Research analyst
(Ankeet.Pandya@MotilalOswal.com)